In a study published in Science Advances, researchers from the University of Cambridge show that tiny components within the cell are the biological engines behind effective protein production.
The endoplasmic reticulum (ER) is the cell’s protein factory, producing and modifying the proteins needed to ensure healthy cell function. It is the cell’s biggest organelle and exists in a web-like structure of tubes and sheets. The ER moves rapidly and constantly changes shape, extending across the cell to wherever it is needed at any given moment.
Using super-resolution microscopy techniques, researchers from Cambridge’s Department of Chemical Engineering and Biotechnology (CEB) have discovered the driving force behind these movements – a breakthrough that could have significant impact on the study of neurodegenerative diseases.
“It has been known that the endoplasmic reticulum has a very dynamic structure – constantly stretching and extending its shape inside the cell,” said Dr Meng Lu, research associate in the Laser Analytics Group, led by Professor Clemens Kaminski.
“The ER needs to be able to reach all places efficiently and quickly to perform essential housekeeping functions within the cell, whenever and wherever the need arises. Impairment of this capability is linked to diseases including Parkinson’s, Alzheimer’s, Huntington’s and ALS. So far there has been limited understanding of how the ER achieves these rapid and fascinating changes in shape and how it responds to cellular stimuli.”
Lu and colleagues discovered that another cell component holds the key – small structures, that look like tiny droplets contained in membranes, called lysosomes.
Lysosomes can be thought of as the cell’s recycling centres: they capture damaged proteins, breaking them down into their original building blocks so that they can be reused in the production of new proteins. Lysosomes also act as sensing centres – picking up on environmental cues and communicating these to other parts of the cell, which adapt accordingly.
There can be up to 1,000 or so lysosomes zipping around the cell at any one time and with them, the ER appears to change its shape and location, in an apparently orchestrated fashion.
What surprised the Cambridge scientists was their discovery of a causal link between the movement of the tiny lysosomes within the cell and the reshaping process of the large ER network.
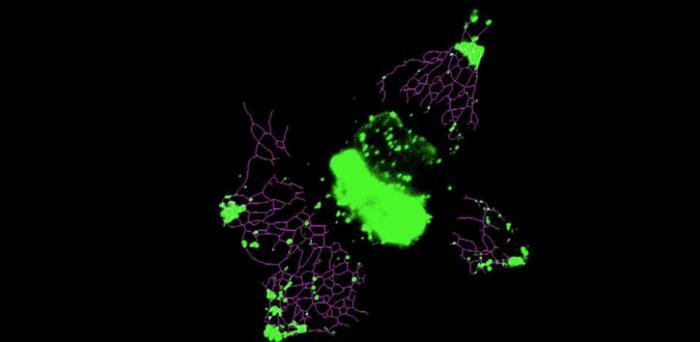
Image: Inducing lysosome (green) anterograde motion with light leads to a rapid and significant extension of ER network (magenta).
Credit: Clemens Kaminski
Reproduced courtesy of the University of Cambridge