These printed drops are naturally trapped at the fluid surface. It is at this point that they are captured as the fluid solidifies around the drops into a solid polymer film. Inspired by the patterns of condensation that form on surfaces, this breakthrough easy-to-manufacture method paves the way for the scaling up of future applications in drug discovery and printed personalised drug delivery.
The Fluids in Advanced Manufacturing research team from the Institute for Manufacturing (IfM), part of the University of Cambridge Department of Engineering, are looking at using the drops as microscale test tubes for reactions. They hope that the millions of drops, capable of fitting on a small area, can be used to accelerate drug discovery reactions. The team will investigate this further in work funded by BBSRC - Biotechnology and Biological Sciences Research Council. Furthermore, the team are exploring the use of capturing and releasing the droplets for tailored treatment of wounds. Working closely with the BBSRC Impact Acceleration Account and University of Cambridge spin-out LIFNanoRx Limited, which uses quantum biology to capture the healing properties of the stem cell growth factor "LIF", the team envisage printed products with the potential value to transform wound healing.
Polymer films with adjustable pores are essential when it comes to designing for applications such as the controlled release of drugs. An example includes the delivery of a personalised combination dosage via a patch or a dissolvable film placed on the tongue. Now the researchers have combined this advanced printing technique with the principles of a nature-inspired method, to provide a manufacturable way of delivering functionality to porous polymer films. The results of the study are published in the journals Materials Horizons and the International Journal of Pharmaceutics.
Natural water condensation patterns that are seen every day on solid surfaces, and which were studied by Lord Rayleigh in 1911, are often referred to as ‘Breath Figures (BFs)’. Since the 1990s, it has been known that these BFs could occur as micron-scale water droplets on a fluid surface, with the ability to self-organise and imprint into a permanent microporous polymer structure. Inspired by this, the Cambridge research team have used drop-on-demand (DoD) inkjet printing to control the size of drops, its contents and location on the fluid surface. In comparison to the BF method, this new process delivers improved stability, with excellent control over pore volume and structure, and enables rapid manufacturing of functional, structured polymer films, making applications feasible and scalable.
The inkjet printing process is highly programmable, with the droplet size and the pattern of the droplets delivered to the substrate easily controlled. The content of the drops can be formulated to contain a wide range of functional materials while still printing reliably. This can include pharmaceutical and biological printing. Each drop is mostly submerged and trapped in the fluid, but with a small opening to the outside. In the first application, for drug discovery, this allows subsequent drops to be added and mixed with drops already on the surface, as if they were microscale test tubes. In the second application, this small opening allows material to be released through diffusion. This allowed researchers Dr Qingxin Zhang and Dr Niamh Willis-Fox to examine each step of the process – print, capture and release. Dr Clare Conboy, from Printed Electronics Ltd., also contributed with expertise and measurements of the behaviour of fluids as they begin to solidify and trap the droplets.
[[{"fid":"292967","view_mode":"default","fields":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":"printing graphic","field_file_image_title_text[und][0][value]":"printing graphic"},"link_text":false,"type":"media","field_deltas":{"1":{"format":"default","alignment":"","field_file_image_alt_text[und][0][value]":"printing graphic","field_file_image_title_text[und][0][value]":"printing graphic"}},"attributes":{"alt":"printing graphic","title":"printing graphic","class":"media-element file-default","data-delta":"1"}}]]
In order to improve drop positioning accuracy, self-organisation was explored as a way of bringing the droplets closer together. This is found to be a highly reliable and repeatable way of ensuring near-perfect droplet packing and the team have been showing how to capture the drops in square arrays or as a hexagonal honeycomb-like structure.
Dr Ronan Daly, Senior Lecturer in the Science and Technology of Manufacturing, said: “This level of control and order has never been achieved with the alternative Breath Figure droplet self-organisation techniques. We have also enabled a shift towards safer, more environmentally responsible manufacturing of these structures. The result is a low cost and customisable technique that has become dramatically more repeatable and tuneable, and one which paves the way for rapid translation to applications in combination drug delivery and drug discovery techniques.”
Dr Su Metcalfe, CEO of LIFNanoRx, said: “The combined powers of printed personalised delivery together with quantum biology of biomimetics, bring a new era of sustainable and universal therapeutics at low cost and high value.”
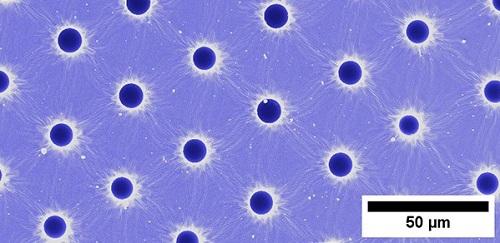
Image (top): Empty pores visible in a polymer film, where droplets were printed, trapped and released.
References:
Qingxin Zhang; Niamh Willis-Fox; Clare Conboy; Ronan Daly. ‘Direct-writing microporous polymer architectures – print, capture and release’. Materials Horizons (2021). DOI: 10.1039/d0mh01460e
Qingxin Zhang; Niamh Willis-Fox; Ronan Daly. ‘Capturing the value in printed pharmaceuticals – A study of inkjet droplets released from a polymer matrix’. International Journal of Pharmaceutics (2021). DOI: 10.1016/j.ijpharm.2021.120436
Reproduced courtesy of University of Cambridge, Department of Engineering