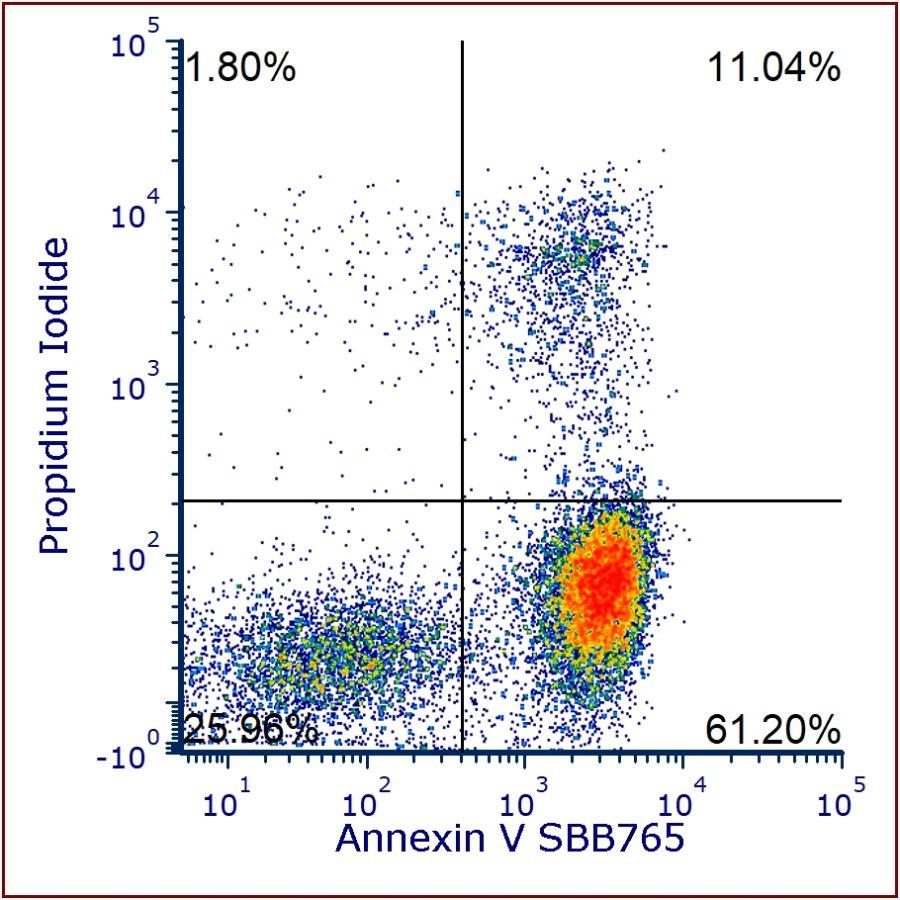
Annexin V StarBright™ Conjugates Offer Increased Choice and Flexibility for Apoptosis Detection in Conventional and Full Spectrum Flow Cytometry
Hercules, Calif. — August 7, 2024 — Bio-Rad Laboratories, Inc., a global leader in life science research and clinical diagnostics products, announces the launch of annexin V conjugated to eight StarBright Dyes: SBUV400, SBUV795, SBV440, SBV515, SBV790, SBB675, SBB765, SBY800. The new Annexin V StarBright conjugates support detection of early apoptotic cells by flow cytometry, offering an increased range of fluorophore options.
Bio-Rad’s range of annexin V conjugates provides researchers with a variety of choices, especially important when including apoptosis detection in multicolor immunophenotyping panels in both conventional and full spectrum flow cytometry. Annexin V StarBright conjugates enable full utilization of all the common laser lines found in flow cytometers such that common viability dye and fluorescent protein emission wavelengths can be avoided. This, combined with the narrow excitation and emission of StarBright Dyes, reduces spillover and spreading to provide high-resolution data.
“Introduction of Annexin V StarBright Dye conjugates offers customers greater choice and flexibility when detecting apoptosis in both conventional and full spectrum flow cytometry,” said Hilary Mavor, Marketing Director, Life Science Group at Bio-Rad Laboratories. “Bio-Rad has one of the most comprehensive ranges of annexin V conjugates, allowing users to follow best practice and accurately detect apoptosis with minimum spillover with their viability dyes.”
Visit bio-rad.com/annexin-v-kit to learn more about Bio-Rad annexin V conjugates.
BIO-RAD, STARBRIGHT are trademarks of Bio-Rad Laboratories, Inc. In certain jurisdictions.
To learn more, visit bio-rad.com.
Forward-Looking Statements
This release may be deemed to contain certain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, without limitation, statements we make regarding our products and our expectations about our products. Forward-looking statements generally can be identified by the use of forward-looking terminology such as "plan", "believe," "expect," "anticipate," "may," "will," "intend," "estimate," "continue," or similar expressions or the negative of those terms or expressions, although not all forward-looking statements contain these words. Such statements involve risks and uncertainties, which could cause actual results to vary materially from those expressed in or indicated by the forward-looking statements. These risks and uncertainties include global economic and geopolitical conditions, our ability to develop and market new or improved products, our ability to compete effectively, international legal and regulatory risks, supply chain risks, and product quality and liability issues. For further information regarding our risks and uncertainties, please refer to the "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operation" in Bio-Rad's public reports filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q. Bio-Rad cautions you not to place undue reliance on forward-looking statements, which reflect an analysis only and speak only as of the date hereof. We disclaim any obligation to update these forward-looking statements.