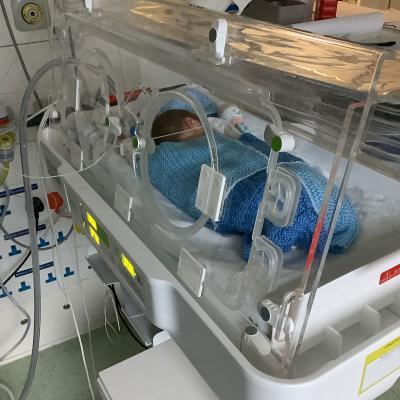
Created by mOm Founder & CEO, James Roberts, and developed by mOm and the engineering team at eg technology, this innovative, lightweight incubator helped to sustain a premature baby’s life at St Peter’s Hospital, Chertsey.
The mOm incubator was originally conceived to save the lives of premature babies in challenging, low/middle-income settings; as well as provide more options for standard neonatal care in the UK and Industrialised economies.
mOm initially approached eg technology to help develop and engineer their concept for a fold-away neonatal incubator. With the requirement that the unit should be designed with accessibility in mind and suitable for use with all major international power voltages, eg technology undertook the entire end-to-end development of the incubator, from initial concept to transfer to manufacture.
eg technology provided mOm with a fully integrated engineering resource, across software, electronics, mechanical engineering and industrial design, working to a clearly defined, phased development programme to meet the clinical and business goals of the client. As with all medical device development, safety and efficacy were central to the programme and eg technology engineers were able to use their expertise to ensurethat the product was developed in accordance with IEC 60601, IEC 62304, ISO 14971, all within eg’s own ISO 13485 quality management system.
eg Director and Co-Founder, Andrew Ede says:"It has been a privilege to be part of this pioneering project. The complexity of the programme, coupled with the development taking place during a global pandemic meant the team had to draw on their extremely broad experience and multi-disciplinary expertise to ensure the project maintained momentum. The regulations around incubator development are, quite rightly, stringent, so to develop a product which met the exacting regulations both from the point of view of device safety and ISO 60601, as well as medical device software to IEC 62304 Class C, is testament to the skill of our engineers."
James Roberts, Founder of mOm, commented:"Sustaining a child's life in our incubator for the first time has been a humbling experience and a monumental step in transforming this dream into a practical reality. It is unacceptable today for one million premature babies to die each year when most of these deaths can be easily prevented. An idea that was once scribbled down on paper now has the potential to impact many lives and I thank the great team at eg technology for helping to turn this dream into a reality."
As a product design engineering and development consultancy, eg technology specialise in taking products from concept to realisation, bridging the gap between innovation and market-ready products. This project utilised the full spectrum of our in-house expertise in software, electronics and mechanical engineering as well as industrial design, human factors, quality assurance and project management.
Please see the full mOm Incubators case study for further information on the design and development of this device.
For more information on getting your technology or ideas to market, or to chat with one of the eg team about your product design and development requirements, please get in touch with one of the eg technology team.
About eg technology
eg technology is a product engineering design and development specialist based in Cambridge. We have extensive experience within the MedTech sector, ranging from microfluidics and diagnostics to surgical instruments and lateral flow. Our engineers are highly skilled in taking devices from concept through to transfer to manufacture. Specialising in electronics, software and mechanical engineering, industrial design, human factors, project management and quality assurance, eg technology is ISO 13485 accredited. We provide our clients with the compliance to standards for safety, quality, risk management and traceability. Our team is able to provide an integrated solution for those who require the expertise to bridge the gap between innovation and a market-ready product.
About mOm
mOm is a MedTech start-up on a mission to provide global access to high-quality healthcare by providing accessible technology solutions that can operate anywhere in the world. The concept for the mOm incubator came to CEO & Founder, James Roberts, in reaction to the appalling infant mortality statistics in low/middle-income countries. In 2014, he won the Sir James Dyson Global Prize for Innovation for his design. The mOm Incubator is a cost-effective, fold-away and lightweight neonatal incubator offering a safe, thermoregulated environment for neonates. It will help reduce the thousands of newborn deaths that occur every day due to a lack of basic medical equipment.
Image credit: Image of infant in Incubator: St Peters Hospital Chertsey with grateful thanks to the parents for their permission to share