The Cambridge Crystallographic Data Centre (CCDC) announces that a published research paper by a team from the CCDC, the University of Leeds, Tianjin University, and AbbVie Inc. has won an Outstanding Manuscript Award from the American Association of Pharmaceutical Scientists (AAPS).
The research paper, published in the scientific journal Pharmaceutical Research, reports investigations into AIDS drug Ritonavir - ‘Solid-State and Surface Structures of the Conformational Polymorphic Forms of Ritonavir in Relation to their Physicochemical Properties.’
“This outstanding paper deserves the full recognition that the AAPS award provides - congratulations to all the scientists involved. We are pleased to have facilitated this scientific research by providing smart, high-quality data and insights from the Cambridge Structural Database (CSD) and the CSD Drug Subset.”
Dr Jürgen Harter, CEO of CCDC.
The work on Ritonavir was part of the Advanced Digital Design of Pharmaceutical Therapeutics (ADDoPT) project, which aimed to develop and implement advanced digital design techniques that can be used in the pharmaceutical industry to deliver medicines more effectively to patients. The winning publication represents an outstanding advancement in underpinning the conformational polymorphism behaviour in Ritonavir and demonstrates the value of large-scale collaborative projects like ADDoPT.
Ritonavir is a drug for the treatment of Acquired Immuno-Deficiency Syndrome (AIDS) marketed in 1996. The discovery of a second, more stable polymorphic form of Ritonavir (form II) within the originally formulated drug product (form I) resulted in a reduction in bioavailability that led to the product’s withdrawal. Interestingly, after its discovery, form II was the only polymorphic form that could be crystallized, while the metastable form I was labelled as a ‘disappearing’ polymorph.
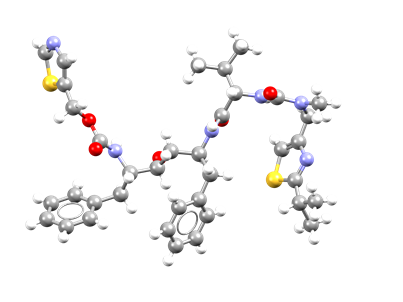
Further studies revealed the presence of five different solid forms for Ritonavir, with only two polymorphs being characterized via single-crystal X-ray diffraction (SCXRD) prior to 2021: the metastable form I and the stable form II. The two polymorphs differ significantly in their molecular conformation, with the most stable form being less dense than the less stable one. A thorough structural investigation was performed for the two forms, but no studies that relate the molecular, solid-state, and surface structure of the two forms were present before the aforementioned study.
The research paper winner of the Outstanding Manuscript Award from the AAPS examines in detail the structure and energetics associated with the polymorphic behaviour of form I and form II of Ritonavir. The work reports a detailed structural characterization of the two polymorphs, a correlation between their structures and lattice energies, and the assessment of the physical-chemical properties of form I and form II.
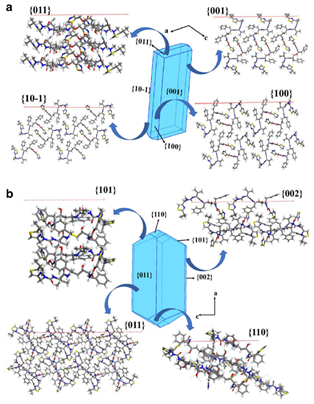
This work highlights how important crystallographic studies are during the development of new drugs. This is critical in understanding and anticipating cases where the solid form of the drug can change to a different polymorphic form within the formulated product.