This post launches a series of infographics focused on pricing and market access trends of rare disease treatments in France. Each post in this series zooms into a specific PMA trend by leveraging RADAR, CRA’s orphan drug data repository that includes all orphan drugs granted EMA approval between July 2013 and September 2023. This first post focuses on time to reimbursement (CT outcome) from EMA approval.
Key takeaways:
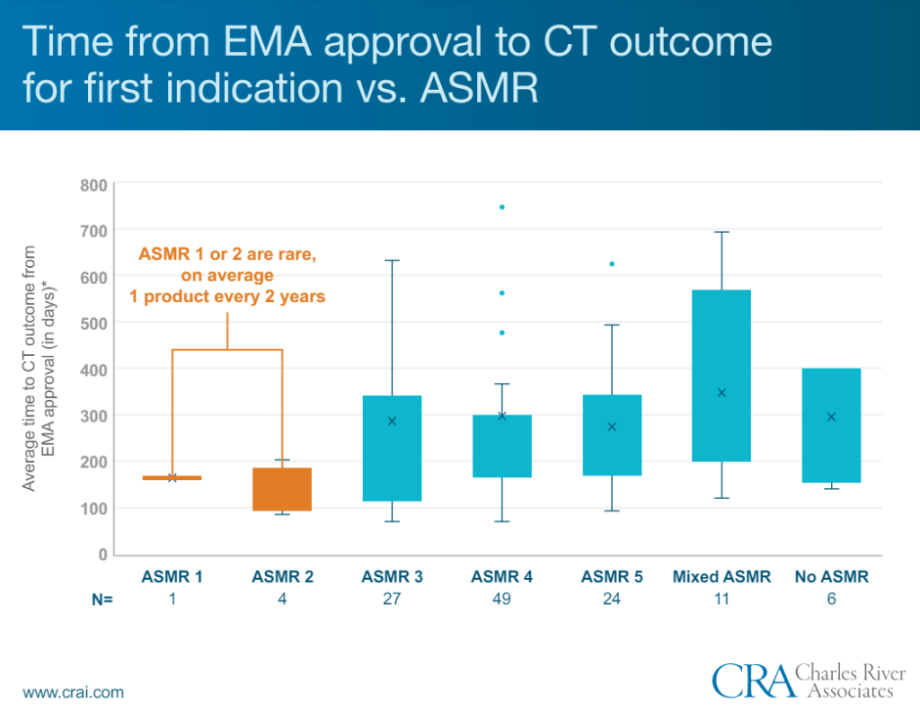
- 5 products are not represented on the graph as time to CT outcome was over 800 days. Whilst Ravicti, Zalmoxis and Jorveza were under the 3-year timeframe, Scenesse and Coagadex took over 5 years to obtain reimbursement
- Only 1 product, Orphacol, has achieved an ASMR 1 since 2013, and none have been granted since 2014. ASMR1 for Orphacol was granted within 160 days of EMA approval, at first submission
- 4 products received ASMR 2, namely Strensiq, Luxturna, Givlaari and Kaftrio. All except Strensiq had secured early access through the ATU prior to receiving reimbursement
32 products overall received an ASMR 3 including 5 as part of a mixed ASMR, an average of 3 per year. Amongst these, 9 received reimbursement restrictions (mixed SMR), including 2 with mixed ASMRs
Click here to read more.