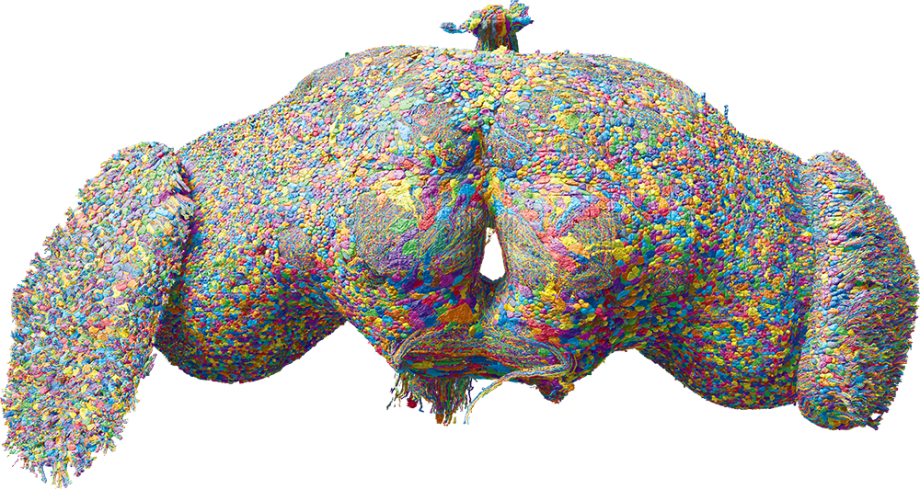An international cohort of researchers called the FlyWire Consortium, including Greg Jefferis’ group in the LMB’s Neurobiology Division and collaborators at Cambridge University, Princeton and the University of Vermont, has joined forces to map every neuron and connection (together known as a connectome) of the fruit fly Drosophila’s brain. The final result is the complete synaptic wiring map of the most complex brain to date. The Drosophila brain contains almost 140,000 neurons and over 15 million synaptic connections. Unlike previously mapped model organisms, the adult fly is capable of complex behaviours, including walking, flying, navigation, singing and complex memory formation, which are reflected in the complexity of its brain.
The finished connectome is reported in a pair of papers in Nature and can be explored online at codex.flywire.ai. The first paper, co-led by Professors Mala Murthy and Sebastian Seung of Princeton University’s Neuroscience Institute, describes the complete proofreading of the connectome in a joint effort between Princeton, Cambridge and researchers around the globe. The second paper, co-led by Greg and Professor Davi Bock at the University of Vermont, systematically annotates the connectome, describing over 8000 unique cell types across the brain, almost 50% more than had been previously proposed to exist in the even more complex mouse brain.
Validating this connectome against a previous partial map, revealed that, contrary to prior concerns, connectomes are not unique ‘snowflakes’ but are instead highly consistent between animals. The exception to this was the finding that around 0.5% of neurons have developmental wiring defects. Similar observations have been made for the connectome of the larval Drosophila brain, published by Marta Zlatic and Albert Cardona’s groups in the LMB’s Neurobiology Division in 2023. The authors suggest that the presence of significant miswiring is true for all complex brains – including humans – and that this could possibly contribute to individuality and differing mental health disorders.
Compiling this complete connectome was a mammoth undertaking. In previous work led by Davi Bock (then at Janelia Research Campus), a single fly brain was sliced into several thousand sections, each measuring only 40 nanometers in thickness. These were then imaged using high resolution electron microscopy in order to resolve fine structures all the way down to individual synapses. The Princeton team then used AI deep neural networks to segment the images and extract neurons and their connections. However, the initial computerised segmentation contained millions of errors requiring manual correction. In total, researchers from Princeton, Cambridge, more than 50 labs around the globe and even a group of citizen scientists collectively spent an estimated 33 years of effort to proofread and correct the segmentation. Once the bulk of the proofreading was complete, Philipp Schlegel and colleagues in the Jefferis group switched to generating a library of over 800,000 annotations to help the many researchers involved in the project navigate the new connectome and cross-reference it to previous results.
These two papers – in combination with many other new publications using FlyWire data – represent a significant step forward in the quest to better understand the inner workings of the brain. Knowing which neurons connect to which other neurons is integral to advancing our knowledge of how brains work. This connectome will allow researchers to easily look up neurons of interest, to see structural connections and form testable hypotheses, which promises to drastically reduce time spent on future neural studies. This connectome also benefits from another key kind of information: the signs of all connections – excitatory vs inhibitory – have been predicted using a new method developed in a collaboration between Greg’s group and Jan Funke at Janelia.
The success of this project illustrates the viability of whole brain mapping for complex creatures, and is likely to be replicated in other organisms such as mice. Finally, Drosophila is a common model for investigating normal brain functions such as memory but also neural disorders, including neurodegenerative diseases. While this dataset was not generated with a specific medical application in mind, the connectome does provide a baseline ‘healthy’ model brain which will no doubt be useful for later comparative studies.
Principal funders for this work include UKRI MRC, Wellcome, the National Institutes of Health (NIH) BRAIN initiative, National Science Foundation (NSF) Neuronex, University of Vermont and Princeton Neuroscience Institute. Proofreading and annotation in Cambridge was carried out in collaboration with the department of Zoology, Ariadne.ai and Aelysia. The Allen Institute for Brain Science provided IT infrastructure.
Read full article below
Image: 3D Rendering of all ~140k neurons in the fruit fly brain, rendered by Philipp Schlegel. Courtesy of MRC Laboratory of Molecular Biology